Answer:
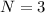
so these are
either 3 to 2 or 2 to 1 or 3 to 1
Step-by-step explanation:
As we know that total number photons emitted from nth state to ground state is given by
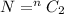
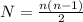
here it shows that if electron has transition from nth excited state to ground state then total number of photons is given by above equation
here we know that higher state is n = 3
so total number of photons is given as
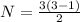
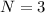
so these are
either 3 to 2 or 2 to 1 or 3 to 1