Answer: 135 grams
Step-by-step explanation:
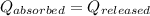
As we know that,
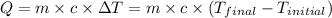
![m_1* c* (T_(final)-T_1)=-[m_2* c* (T_(final)-T_2)]](https://img.qammunity.org/2020/formulas/physics/college/jimhrvet2ab55e8azsefjq8pz22nqyihiz.png)
where,
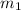 = mass of ice = 100 g
= mass of ice = 100 g
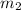 = mass of aluminium cup =? g
= mass of aluminium cup =? g
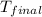 = final temperature =
= final temperature =
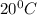
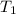 = temperature of ice =
= temperature of ice =
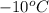
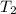 = temperature of aluminium cup=
= temperature of aluminium cup=
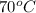
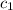 = specific heat of ice=
= specific heat of ice=
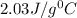
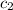 = specific heat of aluminium cup =
= specific heat of aluminium cup =
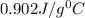
Now put all the given values in equation (1), we get
![[100* 2.03* (20-(-10))]=-[m_2* 0.902* (20-70)]](https://img.qammunity.org/2020/formulas/physics/college/3agelm4g9h8znaqczpf58z7u37dadk1dyf.png)
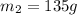
Therefore, the mass of the aluminium cup was 135 g.