Answer:
18931.4
Step-by-step explanation:
Given : velocity of the electron = 2.0
 10
10
mass of the electron = 9.19
 103
103
we know that reduced planks constant, h = 6.5821

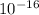 eV s
eV s
We know from uncertainity principle,
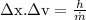
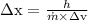
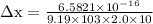
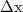 = 18931.4 m
= 18931.4 m
Hence, uncertainty in position of the electron is 18931.4