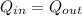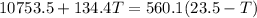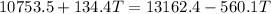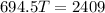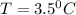Answer:
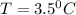
Step-by-step explanation:
Heat given by water + cup = Heat absorbed by the ice
here we can say that let the final temperature of the system is "T"
so we will have heat absorbed by the ice given as
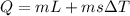
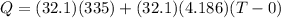
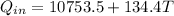
now we will have heat given by cup + water as
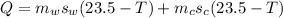
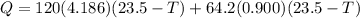
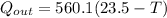
now we have