Answer:
14 electrons can occupy the f sub level
Step-by-step explanation:
Electronic configuration is the short representation which we use to represent the structure of an atom.
Atomic number of phosphorus is 15 so it has 15 electrons.
The electronic configuration of Phosphorus is given as
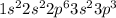
Here the superscript number represents the electrons 2 + 2 + 6 + 2 + 3 = 15 electrons of Phosphorus
Orbital diagram too is used for this purpose.
A circle or a square is used to represent an orbital.
This is the orbital diagram of Radon (atomic number -86)
Each orbital occupies 2 electron.
Shells are named using letters K, L, M, N and so on or using numbers 1, 2, 3, 4 etc.,
The number of electrons, a shell can accommodate is found using formula
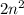 where n is the number of the shell.
where n is the number of the shell.
Shell 1 contains subshell s
Shell 2 contains subshell s, p
Shell 3 contains subshell s, p, d
Shell 4 contains subshell s, p, d, f
Subshells or sub level are represented by letters s, p, d, f, g, h, i and so on.
s contains 1 orbital with 2 electrons
p subshell contains 3 orbital with 6 electrons
d with 5 orbitals and 10 electrons
f with 7 orbitals and 14 electrons.
(Answer)