Answer : The Lewis structures for the two molecules are shown below.
Solution : Given,
If percentage are given then we are taking total mass is 100 grams.
So, the mass of each element is equal to the percentage given.
Mass of C = 52.2 g
Mass of H = 13.1 g
Mass of O = 34.7 g
Molar mass of C = 12 g/mole
Molar mass of H = 1 g/mole
Molar mass of O = 16 g/mole
Step 1 : convert given masses into moles.
Moles of C =
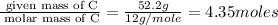
Moles of H =
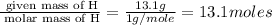
Moles of O =
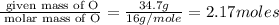
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For C =
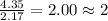
For H =
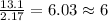
For O =
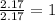
The ratio of C : H : O = 2 : 6 : 1
The mole ratio of the element is represented by subscripts in empirical formula.
The Empirical formula =
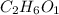 =
=
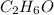
The empirical formula weight = 12(2) + 6(1) + 1(16) = 46 gram/eq
Now we have to calculate the value of 'n'.
Formula used :
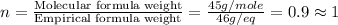
Molecular formula =
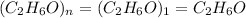
So, there are two possibilities for the arrangements of atoms. That means, it will be an ethanol
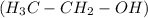 or dimethyl ether
or dimethyl ether
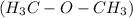 .
.
Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
In the Lewis-dot structure the valance electrons are shown by 'dot'.
As we know that carbon has '4' valence electrons, oxygen has '6' valence electrons and hydrogen has '1' valence electron.
Therefore, the total number of valence electrons in
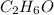 = 2(4) + 6(1) + 6 = 20
= 2(4) + 6(1) + 6 = 20
According to Lewis-dot structure, there are 16 number of bonding electrons and 4 number of non-bonding electrons.
Thus, the Lewis structures for the two molecules are shown below.