Answer:
There are 1.5597 grams of glucose in 100 mL of 0.08658 M glucose solution.
Step-by-step explanation:
13.0 grams of glucose was added to volumetric flask and solution was made up to 100 mL.
Moles of glucose =
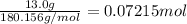
Volume of the solution = 100 mL = 0.1 L
Molarity of the solution =
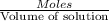
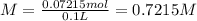
60 mL of 0.7215 M was diluted to 0.500 L, the molarity of the solution after dilution be

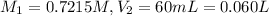
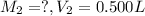
 (Dilution)
(Dilution)
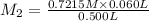
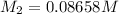
Mass of glucose in 0.08658 M glucose solution:
In 1 L of solution = 0.08658 moles
In 1000 mL of solution = 0.08658 moles
Then in 100 mL of solution :
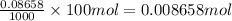 of glucose
of glucose
Mass of 0.008658 moles of glucose:
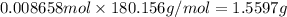
There are 1.5597 grams of glucose in 100 mL of 0.08658 M glucose solution.