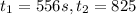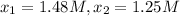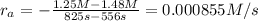Answer:
1) Reaction rate= 0.001230 M/s
2) Reaction rate= 0.001052 M/s
3) Reaction rate=0.000855 M/s
Step-by-step explanation:
Average Rate of the reaction is defined as rate at any instant of time is the rate of change in concentration of any one of the reactants or products with respect to particular time.It is given as;
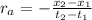
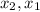 : concentration at time
: concentration at time
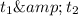 respectively.
respectively.
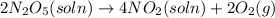
1) Interval: 0 s to 195 s
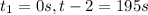
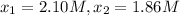
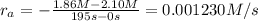
2) Interval: 195 s to 556 s
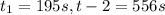
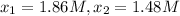
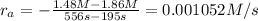
3) Interval: 556 s to 825 s