Answer : The fugacity in the solution is, 16 bar.
Explanation : Given,
Fugacity of a pure component = 40 bar
Mole fraction of component = 0.4
Lewis-Randall rule : It states that in an ideal solution, the fugacity of a component is directly proportional to the mole fraction of the component in the solution.
Now we have to calculate the fugacity in the solution.
Formula used :
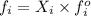
where,
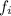 = fugacity in the solution
= fugacity in the solution
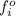 = fugacity of a pure component
= fugacity of a pure component
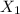 = mole fraction of component
= mole fraction of component
Now put all the give values in the above formula, we get:
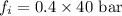
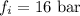
Therefore, the fugacity in the solution is, 16 bar.