Step-by-step explanation:
Charge of electron in He,
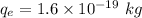
Charge of proton in He,
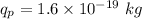
Distance between them,
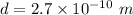
We need to find the electric force between them. It is given by :
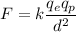
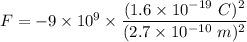
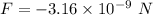
Since, there are two protons so, the force become double i.e.
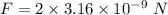
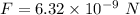
So, the correct option is (c). Hence, this is the required solution.