Answer:
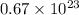 molecules are contained in the flask.
molecules are contained in the flask.
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
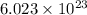 of particles.
of particles.
Standard condition of temperature (STP) is 273 K and atmospheric pressure is 1 atm respectively.
According to the ideal gas equation:'

P= Pressure of the gas = 1 atm
V= Volume of the gas = 2.50 L
T= Temperature of the gas = 273 K
R= Value of gas constant = 0.0821 Latm/K mol
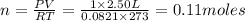
1 mole of gas contains=
 molecules
molecules
0.11 moles of gas contains=
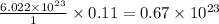 molecules
molecules
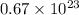 molecules are contained in the flask.
molecules are contained in the flask.