Answer:
The change in internal energy is zero.
(a) is correct option
Step-by-step explanation:
Given that,
Work done W = -400 J
The First law of thermodynamics is law conservation of energy
Law of conservation energy is defined the total amount of energy is constant in isolated system.
The energy can not be created and destroyed but the energy can be changed from one form to other form.
The First law of thermodynamics is given by
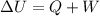
Where,
W = work done by the system or on the system
 = Total internal energy
= Total internal energy
Q = heat
In isothermal process, the temperature is constant
The energy can not be change and the internal energy is the function of temperature.
So, The internal energy will be zero .
Hence, The change in internal energy is zero.