Answer: The pH of Lake B is 5.
Step-by-step explanation:
pH is defined as negative logarithm of hydrogen ion concentration. It is basically defined as the power of hydrogen ions in a solution.
Mathematically,
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
We are given:
Hydrogen ion concentration of Lake A,
![[H_A]=1* 10^(-6)M](https://img.qammunity.org/2020/formulas/chemistry/college/i34250r15ldg0g0qhpam8sb66g3m8bvsq6.png)
Hydrogen ion concentration of Lake B,
![[H_B]=1* 10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/college/jwtoc2ixcb9dy6pujp9rtxtr864g4vhewk.png)
Hydrogen ion concentration of Lake C,
![[H_C]=1* 10^(-4)M](https://img.qammunity.org/2020/formulas/chemistry/college/8mm2egu7o0o8xmv7eobxhzgca3nwx03t8y.png)
Putting values of hydrogen ion concentration for Lake B in above equation, we get:
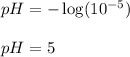
Hence, the pH of Lake B is 5.