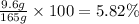Answer:
The concentration of sodium bromide solution as a percent by mass is 5.82%.
Step-by-step explanation:
Percent by Mass (w/w %) :
The percentage mass or fraction of mass of the of solute present in total mass of the solution.
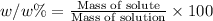
Mass of solute that is sodium bromide = 9.6 g
Mass of the solution prepared = 165 g
The concentration of solution as a percent by mass: