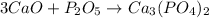Step-by-step explanation:
1) When phosphoric acid reacts with calcium hydroxide neutralization reaction takes place in which calcium phosphate is formed along with formation of water.
The balanced chemical equation is give as:
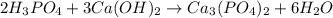
2) When calcium oxide acid reacts with phosphorus pentoxide addition reaction takes place in which calcium phosphate is formed.
The balanced chemical equation is give as: