Answer:
The new volume of this gas is 6.86 liters.
Assumption: the temperature of this gas stays the same, and this gas is ideal such that Boyle's Law applies.
Step-by-step explanation:
By Boyle's Law, the volume of an ideal gas shall be inversely proportional to the pressure on it when temperature stays the same (as in an isothermal process.)
In other words,
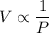 ,
,
where
 is the volume of the gas, and
is the volume of the gas, and
 is the pressure on the gas.
is the pressure on the gas.
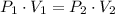 .
.
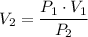 .
.
Assume that this gas is ideal. Also assume that this increase in pressure is isothermal. Apply Boyle's Law to find the new volume of this gas:
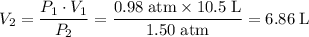 .
.