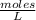Answer:
The molarity of the solution is 0.05
Step-by-step explanation:
Molarity (M) is the number of moles of solute that are dissolved in a given volume.
Molarity is determined by the following expression:
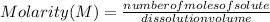
Molarity is expressed in units
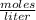 .
.
Then you should know the amount of moles of HCL. For that you should know that:
The Avogadro Number or Avogadro Constant is called the number of particles that constitute a substance (usually atoms or molecules) and that can be found in the amount of one mole of that substance. Its value is 6.023 * 10²³ particles per mole. Avogadro's number applies to any substance.
Therefore, applying a rule of three, 6.02*10²² molecules correspond to:
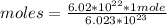
moles=0.1 moles of HCl
Applying the definition of molarity:
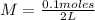
M= 0.05
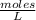
The molarity of the solution is 0.05