Answer:
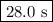
Step-by-step explanation:
Whenever a question asks you, "How long does it take to reach a certain concentration?" or something like that, you must use the appropriate integrated rate law expression.
The integrated rate law for a first-order reaction is
![\ln \left (([A]_(0))/([A]) \right ) = kt](https://img.qammunity.org/2020/formulas/chemistry/college/sglj8ijowbjescmf14erbxxh6ercvvod0l.png)
Data:
[A]₀ = 1.28 mol·L⁻¹
[A] = 0.17 [A]₀
k = 0.0632 s⁻¹
Calculation:
![\begin{array}{rcl}\ln \left (([A]_(0))/(0.170[A]_(0)) \right ) & = & 0.0632t\\\\\ln \left (5.882) & = & 0.0632t\\1.772 & = & 0.0632t\\\\t & = & (1.772)/(0.0632)\\\\t & = & \textbf{{28.0 s}}\\\end{array}\\\text{It will take } \boxed{\textbf{28.0 s}} \text{ for [HI] to decrease to 17.0 \% of its original value.}](https://img.qammunity.org/2020/formulas/chemistry/college/8fdtbonxy5f25f0z3g7s1sab00hec0grcw.png)