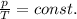Answer:
All these laws give the relationship between two quantities of the gas among V (volume), p (pressure) and T (temperature), keeping the third one constant - however the two quantities change for each law
Step-by-step explanation:
Calling:
p = gas pressure
V = gas volume
T = gas temperature (in Kelvin)
We have:
- Boyle's law: the pressure and the volume of a gas kept at constant temperature are inversely proportional. Mathematically,
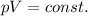
- Charles's law: the temperature and the volume of a gas kept at constant pressure are directly proportional. Mathematically,
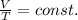
- Gay Lussac's law: the temperature and the pressure of a gas kept at constant volume are directly proportional. Mathematically,