Step-by-step explanation:
Energy necessary to remove an electron from outer orbital of a neutral gaseous atom or molecule is known as ionization energy.
Bohr's equation to calculate energy is as follows.
![\Delta E = R_(H)[(1)/(n^(2)_(i)) - (1)/(n^(2)_(f))]](https://img.qammunity.org/2020/formulas/chemistry/high-school/yile44orbs71fhdsp29wnfozwx8bt0kbh2.png)
where,
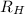 = Reydberg's constant =
= Reydberg's constant =
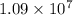 per meter
per meter
In the given case,
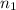 = 1 and
= 1 and
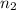 =
=
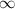
Therefore, calculate the energy as follows.
![\Delta E = R_(H)[(1)/(n^(2)_(i)) - (1)/(n^(2)_(f))]](https://img.qammunity.org/2020/formulas/chemistry/high-school/yile44orbs71fhdsp29wnfozwx8bt0kbh2.png)
=
![1.09 * 10^(7)[(1)/((1)^(2)) - (1)/(\infty)}]](https://img.qammunity.org/2020/formulas/chemistry/high-school/kihgg1rjx49mdewsl43l6308tf7rb0eddn.png) J
J
=
![1.09 * 10^(7)[1 - 0}]](https://img.qammunity.org/2020/formulas/chemistry/high-school/c7ez9nhsvcdze4vgbzsivcxczgc1d8pyj4.png) J
J
=
![1.09 * 10^(7)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/5ha6aqm7buyh1fozhpwiqrnhgn8dflysvg.png) J
J
As atomic configuration of helium is
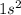 . Thus, energy required to produce
. Thus, energy required to produce
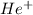 and
and
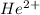 will be the same.
will be the same.
Also, 1 joule = 0.239 calories. Hence, convert calculated energy into joules as follows.
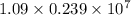 J
J
=
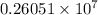 cal
cal
=
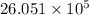 cal
cal
Therefore, we can conclude that
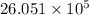 cal energy is required to produce, from neutral He atoms,1 mole of
cal energy is required to produce, from neutral He atoms,1 mole of
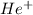 ions and
ions and
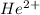 ions using Bohr's equations.
ions using Bohr's equations.