Answer: Option (b) is the correct answer.
Step-by-step explanation:
It is known that number of moles of a substance is equal to the given mass of the substance divided by its molar mass.
Mathematically, Number of moles =
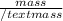
Molar mass of beryllium is 9 g/mol. Hence, putting the given values into the above formula as follows.
Number of moles =
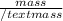
=
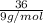
= 4 moles
Thus, we can conclude that the number of moles present in 36 grams of Be are 4 moles.