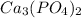Answer : The formula for the compound is
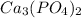
Explanation :
For formation of a neutral ionic compound, the charges on cation and anion must be balanced.
The cation is formed by loss of electrons by metals and anions are formed by gain of electrons by non-metals.
In the given question, the calcium metal forms an ionic compound with polyatomic anion (phosphate)
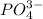 .
.
Here, calcium is having an oxidation state of +2 called as
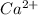 cation and phosphate
cation and phosphate
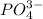 is an anion with oxidation state of -3. The charges are not balanced. So, the charges are balanced by the cris-cross method. Thus, the compound formed will be,
is an anion with oxidation state of -3. The charges are not balanced. So, the charges are balanced by the cris-cross method. Thus, the compound formed will be,
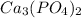
Hence, the formula for the compound is