Answer:
Step-by-step explanation:
The reaction between propane and oxygen is a combustion process for an hydrocarbon.
The formula of propane is given as C₃H₈ and oxygen is written as O₂
For this combustion reaction, the general equation is given as:
Cₓ
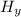 + (x +
+ (x +
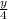 )O₂ → xCO₂ +
)O₂ → xCO₂ +
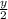 H₂O
H₂O
In the reaction between propane and oxygen,
x = 3
y = 8
The reaction is then written as:
C₃H₈ + (3 +
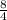 )O₂ → 3CO₂ +
)O₂ → 3CO₂ +
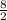 H₂O
H₂O
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O