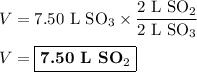Answer:
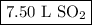
Step-by-step explanation:
For this problem, we must use Gay-Lussac's Law of Combining Volumes:
The ratio in which gases react is the same as the ratio of their coefficients in the balanced equation.
The balanced equation is
Theor: 2 L 2 L
2SO₂ + O₂ ⟶2 SO₃
V/L: 7.50
Gay-Lussac's Law tells us that 2 L of SO₂ form 2 L of SO₃.
The volume of SO₂ used was