Answer:
Diamond and graphite are allotropes of carbon.
Step-by-step explanation:
Diamond has a close packed crystal structure which is responsible for its extreme hardness. In it, each carbon atom is sp³ hybridised and bonded to four other carbon atoms in a tetrahedral arrangement. Diamond has a hardness of 10 on mohs scale with a cubic crystal form.
Note: allotropes of an element have different molecular structure.
Graphite on the other hand is made up of layers of hexagonal structure that are weakly bonded by van-der-waals forces. This layered arrangement explains its softness/slippery feeling and hence it is used as a lubricant. In each layer, each carbon atom is sp² hybridised and bonded to three other carbon atoms in a trigonal planar arrangement.
The presence of
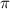 electrons in the layers accounts for its ability to conduct electricity.
electrons in the layers accounts for its ability to conduct electricity.