Answer:
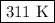
Step-by-step explanation:
We can use the Ideal Gas Law and solve for T.
pV = nRT
Data:
p = 0.998 atm
V = 1.20 L
n = 0.0470 mol
R = 0.082 06 L·atm·K⁻¹mol⁻¹
Calculation:
0.998 × 1.20 = 0.0470 × 0.082 06 × T
1.198 = 0.003 857T
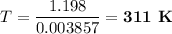
The Kelvin temperature is
 , not 307 K.
, not 307 K.
I suppose you could choose the last square, as it has the closest value.