Answer:
The molarity of a solution that contains 8 moles of NaOH in 0.5 liters of solution is 16.0 M.
Step-by-step explanation:
Molarity of the solution is the moles of compound in 1 Liter solutions.
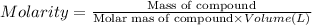
Moles of NaOH = 8 moles
Volume of the solution = 0.5 L
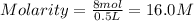
The molarity of a solution that contains 8 moles of NaOH in 0.5 liters of solution is 16.0 M.