Answer: Option (b) is the correct answer.
Step-by-step explanation:
A reaction equation is an equation in which reactants are placed on the left side whereas products are placed on the right side.
For example,
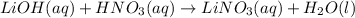
Here, lithium hydroxide on reaction with nitric acid leads to the formation oof lithium nitrate and water.
It is a neutralization reaction, as base is reacting with an acid.
Therefore, we can conclude that products for the given reaction are
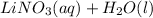 .
.