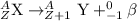Answer:
Explanation:-
1. Activation energy is the extra energy required by the reactants to cross the energy barrier and convert to products. It is designated by the symbol
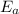 .
.
2. Half life is the time taken by a reactant to reduce to its original concentration. It is designated by the symbol
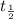 .
.
3. Alpha decay: In this process, alpha particles is emitted when a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units.
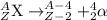
4. Beta-decay: In this process, a neutron gets converted into a proton and an electron releasing a beta-particle. The beta particle released carries a charge of -1 units.