Answer:
The correct answer is option C.
Step-by-step explanation:
Metallic sulfides on combustion gives metallic oxides along with sulfur dioxide as product.During combustion reaction oxygen reacts with compound.
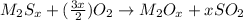
So, when compound of containing metal and sulfur undergone combustion reaction it gives compound X and compound Y.
Compound X contains metal atom which means it is metal oxide.
Compound Y contains sulfur atom which means it is sulfur oxide.