Answer: Option (d) is the correct answer.
Step-by-step explanation:
A reaction in which heat is absorbed by the molecules of a substance is known as an endothermic reaction.
For example, when ice melts then molecules of ice gain kinetic energy due to which they tend to move apart from each other.
As a result, solid state changes into liquid state.
Also, it is known that entropy is the degree of randomness. So, increase in kinetic energy of a substance tends to increase entropy.
As kinetic energy is proportional to temperature through the relation as follows.
K.E =
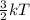
Therefore, we can conclude that the statement high temperature only, because entropy increases during melting best describes the temperature conditions that are likely to make this a spontaneous change.