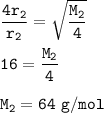The relative molecular mass of the gas : 64 g/mol
Further explanation
Given
Helium rate = 4x an unknown gas
Required
The relative molecular mass of the gas
Solution
Graham's Law
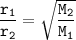
r₁=4 x r₂
r₁ = Helium rate
r₂ = unknown gas rate
M₁= relative molecular mass of Helium = 4 g/mol
M₂ = relative molecular mass of the gas
Input the value :