Answer:
D. Temperature
Step-by-step explanation:
The temperature of a substance is directly proportional to the average kinetic energy of the particles in the substance according to the equation (valid for monoatomic gases)
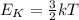
where
Ek is the average kinetic energy
k is the Boltzmann's constant
T is the temperature
From the equation, we see that the temperarure is directly proportional to the average kinetic energy, so the correct answer is
D. temperature