18. When its outermost shell is completely full
Explanation: an atom is unlikely to react when its outermost orbital is completely full with electrons. The atoms of these kind of elements are called noble gas, and they are unlikely to react with other elements because they do not accept electrons from other atoms and they do not give their electrons to other atoms, since their outermost shell is already full. Examples of noble gases are helium, neon and argon.
19. Types of nuclear radiation from lowest to highest energy:
- Beta radiation: beta particles are fast-moving electrons or positrons emitted in beta decay of the nuclei, and their energy typically ranges from a few hundreds keV to a few MeV
- Alpha radiation: alpha particles are nuclei of helium emitted in alpha decays of the nuclei, and their energy is typically of 5 MeV
- Gamma radiation: these are gamma ray photons emitted from nuclei in excited state that decay back into the lowest energy state. Typical energy of gamma radiation are a few MeV.
20. The false statement is C
In fact, it's actually the opposite: fission and fusion are two processes that release huge amount of energy, according to Einstein's famous equation

where E is the energy released,
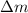 the variation of mass between initial and final products, and c is the speed of light. Given the huge value of
the variation of mass between initial and final products, and c is the speed of light. Given the huge value of
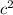 , we see that even for a tiny mass difference the amount of energy released in these processes is very big.
, we see that even for a tiny mass difference the amount of energy released in these processes is very big.
21. Uranium-235 and uranium-238
Uranium-238 is the naturally occuring radioisotope used in the nuclear reactors. However, since it is not fissile, it is enriched with another isotope, the uranium-235, which is fissile and therefore can substain the chain reaction in the nuclear reactor.
22. Because it is written as first element
In the chemical formula of a ionic compound (such as
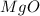 ), the first element is the cathion (positive ion) while the second one is the anion (negative ion). This means that the magnesium (Mg) is the element with one electron in excess (therefore, it is the metal, since metals are the element with free electrons), while oxygen (O) is the non-metal, since it actually has 1 vacancy in its outermost shell.
), the first element is the cathion (positive ion) while the second one is the anion (negative ion). This means that the magnesium (Mg) is the element with one electron in excess (therefore, it is the metal, since metals are the element with free electrons), while oxygen (O) is the non-metal, since it actually has 1 vacancy in its outermost shell.
23. 19: atomic number, 39.098: atomic mass
There are two numbers that characterize an element:
- atomic number (indicated with Z): it is the number of protons in the nucleus --> for potassium, Z=19, since it contains 19 protons
- mass number (indicated with A): it is the number of protons+neutrons in the nucleus, and it is calculated as an average between the different isotopes of the element. In this case, A=39.098, which means that on average potassium contains 39.098 protons+neutrons
24: X and Z have similar properties
The chemical properties of an element are determined by the number of valence electrons that it has: in fact, this number determines how much the element tends to react with other elements.
Elements that have the same number of valence electrons, therefore, react with other elements in a similar way, so they also have similar properties.