Answer:
Q = 78375 [J]
Step-by-step explanation:
To solve this problem we must use the following ideal equation for the thermal energy of an element or substance depending on the temperature change.
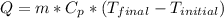
where:
Q = heat [J]
m = mass = 0.25 [kg]
Cp = specific heat = 4180 [J/kg*C]
Tinitial = 25[°C]
Tfinal = 100 [°C]
![Q =0.25*4180*(100-25)\\Q = 78375 [J]](https://img.qammunity.org/2022/formulas/physics/high-school/8nzc2mjh6g9itq5mxfqtjtd5axbmpbuigb.png)