Answer: The correct answer is Option E.
Step-by-step explanation:
Solubility is defined as the amount of substance (known as solute) which is dissolved in a unit volume of liquid substance (known as solvent) to produce a saturated solution at specific temperature and pressure. It is expressed in number of moles of solute present per 100 grams of solvent.
This property is directly dependent on temperature. As, the temperature increases, the solubility of the solute increases and vice-versa.
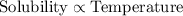
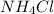 is an ionic salt whose solubility increases as we increase the temperature.
is an ionic salt whose solubility increases as we increase the temperature.
The solubility of ammonium chloride at 50°C is 50 grams.
Hence, the correct answer is Option E.