Answer:Volume occupied by the gas at standard pressure is 162.5 L.
Step-by-step explanation:
Pressure of the gas when it occupies volume of 190 L = 650 mmHg
Volume occupied by the gas when the gas is at standard pressure.
The value of standard pressure = 1 atm = 760 mmHg
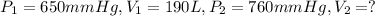
According to Boyle's Law:
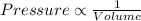 (at constant temperature)
(at constant temperature)
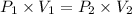
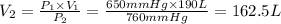
Volume occupied by the gas at standard pressure is 162.5 L.