Answer: Option (B) is the correct answer.
Step-by-step explanation:
The given data is as follows.
mass = 0.755 g, Q = 0.954 J
 = ? , Specific heat of water, C =
= ? , Specific heat of water, C =
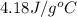
As the relation between heat energy, mass and change in temperature are as follows.
Q =
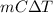
0.954 J =
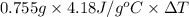
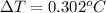
Thus, we can conclude that increase in temperature is
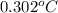 .
.