Answer:
The correct answer is option C.
Step-by-step explanation:
Oxidation number : The charge on the central atom calculated by assigning charges to other atoms or ligands and putting their sum equal to the overall charge on the ion or molecule or coordination sphere.
1) Oxidation state of nitrogen atom in
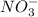 ion:
ion:
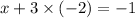
x = 5
2) Oxidation state in
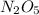
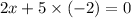
x = +5
3) Oxidation state in
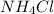
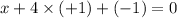
x = -3
4) Oxidation state in
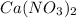
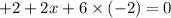
x = +5
Hence, the correct answer is option C.