Answer:
Q = 675 [J]
Step-by-step explanation:
We can calculate the amount of heat transfer by means of the following expression that includes the mass and temperature change in a body as a function of the specific heat.
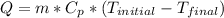
where:
m = mass = 25 [gr]
Cp = specific heat = 0.9 [J/g*°C]
Tinitial = 55 [°C]
Tfinal = 25 [°C]
![Q=25*0.9*(55-25)\\Q=675 [J]](https://img.qammunity.org/2022/formulas/physics/college/hl9h7hfzjciv7eqvaayk24c1xkg0q6yh5w.png)