Explanation :
Solution for part (a) :
Concentration of
 =
=
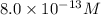
As we know that,
![[H^+][OH^-]=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/kygctthkoa5ws7izf8efhkrnwy3gl37uak.png)
Now put the value of concentration of hydrogen ion, we get concentration of hydroxide ion.
![(8.0* 10^(-13))* [OH^-]=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/41fi3hxfaw9aw5xpn68l1bs8qaofxnkos3.png)
![[OH^-]=1.25* 10^(-2)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/fvp4ayouqc1blch9cyy6su5uvqtz9sq3md.png)
From this we conclude that, the concentration of hydroxide ion is more than the hydrogen ion that means the solution is basic in nature.
Solution for part (b) :
Concentration of
 =
=
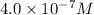
As we know that,
![[H^+][OH^-]=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/kygctthkoa5ws7izf8efhkrnwy3gl37uak.png)
Now put the value of concentration of hydroxide ion, we get concentration of hydrogen ion.
![[H^+]* (4.0* 10^(-7))=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/m5nclzqq4dsk95ngfx79coqp9r6p72y44e.png)
![[H^+]=0.25* 10^(-7)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/k3g6s34t1pmwsvcso1gzfm5w90whb82z9e.png)
From this we conclude that, the concentration of hydrogen ion is more than the hydroxide ion that means the solution is acidic in nature.
Solution for part (c) :
Concentration of
 =
=
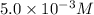
As we know that,
![[H^+][OH^-]=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/kygctthkoa5ws7izf8efhkrnwy3gl37uak.png)
Now put the value of concentration of hydroxide ion, we get concentration of hydrogen ion.
![[H^+]* (3.0* 10^(-3))=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/v49oha0edevzxm8blmvbva0ezvm8yuwxyt.png)
![[H^+]=0.25* 10^(-7)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/k3g6s34t1pmwsvcso1gzfm5w90whb82z9e.png)
From this we conclude that, the concentration of hydroxide ion is more than the hydrogen ion that means the solution is basic in nature.
Solution for part (d) :
Concentration of
 =
=
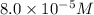
As we know that,
![[H^+][OH^-]=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/kygctthkoa5ws7izf8efhkrnwy3gl37uak.png)
Now put the value of concentration of hydrogen ion, we get concentration of hydroxide ion.
![(8.0* 10^(-5))* [OH^-]=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/pesu9o6bg4gi28ywjoh1d0wtgqeknj7rmp.png)
![[OH^-]=1.25* 10^(-2)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/fvp4ayouqc1blch9cyy6su5uvqtz9sq3md.png)
From this we conclude that, the concentration of hydrogen ion is more than the hydroxide ion that means the solution is acidic in nature.