This problem can be solved using the Combined Gas Law. The formula for it is
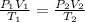
Let's go ahead and plug in the known values. Since pressure remains the same, it does not matter what value we plug in, so let's assume 1 atm for both sides.
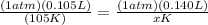
Now, we just need to solve for the unknown. Start by simplifying the left side and right numerator.
0.001 =
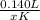
Multiply both sides by x.
0.001x = 0.140
Divide both sides by 0.001.
x = 140 K
Convert that to Celsius (K = C + 273)
140 = C + 273
C = -133
So, the volume of your gas will be 0.140 L at -133° C.