Answer:
The final volume of the Nitrogen gas is 54.03 L.
Step-by-step explanation:
Given;
initial volume of the Nitrogen gas, V₁ = 80 L
initial pressure of the Nitrogen gas, (at STP), P₁ = 101.3 kPa
final pressure of the Nitrogen gas, P₂ = 150 kPa
If the temperature is held constant, apply Boyle's law to determine the final volume of the Nitrogen gas.
V₁P₁ = V₂P₂
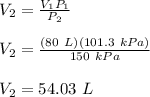
Therefore, the final volume of the Nitrogen gas is 54.03 L.