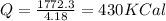As we know that in order to melt the copper we need to take the temperature of copper to its melting point
So here heat required to raise the temperature of copper is given as
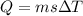
We know that
melting temperature of copper = 1085 degree C
Specific heat capacity of copper = 385 J/kg C
now we have
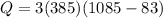
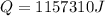
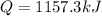
now in order to melt the copper we know the heat required is
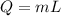
here we know that
L = 205 kJ/kg
now from above formula
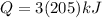
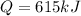
now total heat required will be
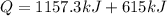
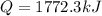
As we know that
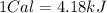
now we have