PART A)
As we know that total number of neutrons and protons will remains conserved in both sides of the reaction
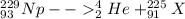
so here atomic number is 91 and mass number is 225
so this atom must be Pa
so it is
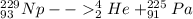
PART B)
As we know that total number of neutrons and protons will remains conserved in both sides of the reaction
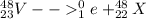
so here atomic number is 22 and mass number is 48
so this atom must be Ti
so it is
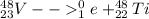
PART C)
In this given figure we can see that a big and heavy nucleus is broken into smaller nuclei
This type of nuclear reactions are known as Fission reaction where a big unstable nuclei will convert into smaller stable nuclei
In this process of stability the energy is released and that is used in form of kinetic energy and electromagnetic radiation energy