Answer:
31.38 °C will be the final temperature of the system.
Step-by-step explanation:
a) Heat lost by iron will be equal to heat gained by the water
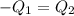
Mass of iron =
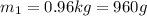
Specific heat capacity of iron =
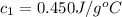
Initial temperature of the iron =
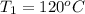
Final temperature =
 =T
=T
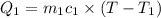
Mass of water=
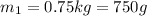
Specific heat capacity of water=
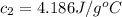
Initial temperature of the water =
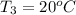
Final temperature of water =
 =T
=T
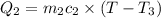
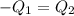
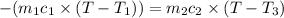
On substituting all values:
we get, T = 31.38 °C
31.38 °C will be the final temperature of the system.
b) During an endothermic reaction, energy is absorbed from the surroundings and temperature of the surrounding decreases.The energy of the reactants are less than that of the energy of the products.
If the reaction is an endothermic , then the surrounding that is beaker will cool down due to lowering in lower in temperature.This lowering down of temperature is due to the absorption of kinetic energy of the particles in the system by products.
c) When the substance is freezes its potential energy increases . Energy is released when substance freezes and this energy is termed as latent heat of freezing.
Also with decrease in temperature of the of substance the kinetic energy of the particles decreases as we know that temperature and kinetic energy are directly related. And with decrease in kinetic energy the potential energy of the substance particle will increase as per as law of conservation of energy.