Answer:

Step-by-step explanation:
Molarity, which tells us the concentration of a solution, is found by dividing the moles of solute by the liters of solution.
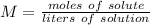
1. Define Values
There are 3.0 Moles of NaCl. This is the moles of solute.
There are 250 milliliters of solution, but we need the liters.
a. Convert mL to L
1 milliliter is equal to 0.001 liters. We can multiply the given number of milliliters (250) by 0.001.
250 mL * 0.001 L/mL= 0.25 L
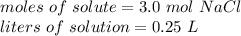
2. Calculate Molarity
Substitute the values into the formula.
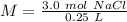
Divide.
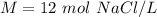
3. Define Units
1 mole per liter is equal to 1 molar.
Our answer of 12 mol NaCl/ L is equal to 12 M NaCl
The molarity is 12 M NaCl