Answer:
The parent isotope remain after 20.25 hours will be 6.25 grams.
Step-by-step explanation:
Initial mass of the Pa-234 isotope = 50.0 g
Time taken by the sample, t = 20.25 hr
Formula used :
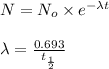
where,
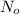 = initial mass of isotope
= initial mass of isotope
N = mass of the parent isotope left after the time, (t)
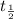 = half life of the isotope = 6.75 hr
= half life of the isotope = 6.75 hr
 = rate constant
= rate constant
Now put all the given values in this formula, we get
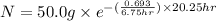
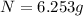
Therefore, the parent isotope remain after 20.25 hours will be 6.25 grams.