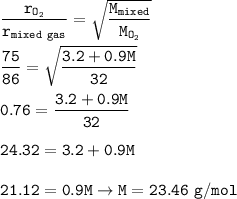
The molecular weight of unknown gas : 23.46 g/mol
Further explanation
Given
A vessel contains 10% of oxygen and 90% of an unknown gas.
diffuses rate of mixed gas = 86 s
diffuses rate of O₂ = 75 s
Required
the molecular weight of unknown gas (M)
Solution
The molecular weight of mixed gas :(M O₂=32 g/mol)
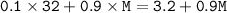
Graham's Law :