Answer: The correct answer is Option A.
Step-by-step explanation:
Fluorine is the 9th element in the periodic table which belongs to group 17 and period 2. It is a non-metal because it requires an electron to gain its stable electronic configuration.
The electronic configuration of this elements is:
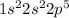
This element requires 1 electron to attain stable configuration. It is an insulator and is not lustrous.
This element easily gains an electron and hence, is considered as a highly reactive non-metal.
Hence, the correct answer is Option A.